Coordination complexes as catalysts for Chan-Evans-Lam couplings

The Chan-Evans-Lam coupling of boronic acids with nucleophiles provides a heavy metal-free alternative to Buchwald-Hartwig couplings and operates under milder reaction conditions. Most commonly employed catalysts are simple copper salts, such as copper acetate. Chan-Evans-Lam couplings are notorious for being highly substrate dependent, making laborious optimization of reaction conditions necessary even for closely related substrates such as aliphatic amines and anilines.
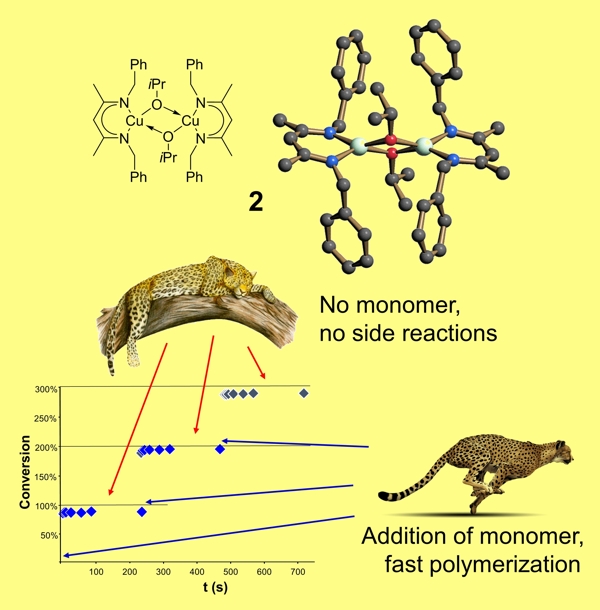
Among the factors optimized are the solvent, the base/ligand added, the copper source (counter anion), elimination of water etc. We speculated that incorporating the necessary functionalities typically attributed to solvent, base and/or anion into the ligand framework of a coordination complex would result in a more general coupling protocol. In fact, complex 1 is one of the most active catalyst for Chan-Evans-Lam couplings and couples a large variety of N-nucleophiles using an identical, simple reaction protocol, tolerating the presence of water. None of the common site reactions in Chan-Evans-Lam couplings are observed and excess of boronic acid is not required. This catalyst is particularly active towards sterically hindered amines.
Selected publications:
73. [ACS Editors' Choice] Chan–Evans–Lam Couplings with Copper Iminoarylsulfonate Complexes: Scope and Mechanism
69. Sulfonato-imino copper(II) complexes : fast Chan-Evans-Lam coupling of amines and anilines
66. [Website Banner Choice] Sulfonato-diketimine copper(II) complexes : synthesis and application as catalysts in base-free Chan-Evans-Lam couplings