
 |

|
3. The Electron Acceptor Site:The secondary electron acceptors in PSII are quinone-based molecules,
Qa, which remains in the vicinity of the reaction centre, and Qb, which
diffuses out of PSII after being reduced (Fig. 2). Most artificial systems
attempt to mimic Qa as it is synthetically easy to attach a quinone to
a chromophore.17 A much
more challenging approach, and one more directly related to Qb, is to
link the chromophore to a receptor that binds quinone non-covalently.
Very few systems of this sort exist,18
even though the diffusion of the reduced form of Qb away from the reaction
centre is critical to avoid charge recombination. We are modelling quinone
electron acceptors using metalloreceptors that bind substrate molecules
non-covalently. 3.1 The design of receptors for the non-covalent binding of electron acceptors |
 |
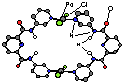 |
Figure 11 Synthesis and X-ray crystal structure of a dipalladium receptor (DMSO omitted).
Thus, metal-directed self-assembly is a viable approach to receptor synthesis. However, this charge neutral complex has limited solubility in common organic solvents. By using neutral ligands and forming charged complexes we were able to solubilize these complexes in a wide range of solvents (Fig. 12).
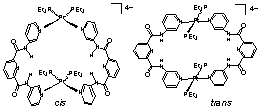
Figure 12 Cis and trans diplatinum receptors
In the presence of cis platinum macrocycle, we have shown that quinone is reduced to hydroquinone. We are interested in using quinone/hydroquinone as an electron trap, e.g., if the phosphine cone angles in trans complexes are increased, can quinone be non-covalently bound in the metallomacrocycles? As membrane and protein environments are known to modify the electro- and photochemical properties of molecules, we would like to do the same within this small metalloreceptor nano-environment. As supramolecular catalysis is rare,19 we are investigating the mechanism of this reduction more closely.