
|

|

1. Artificial Photosynthesis
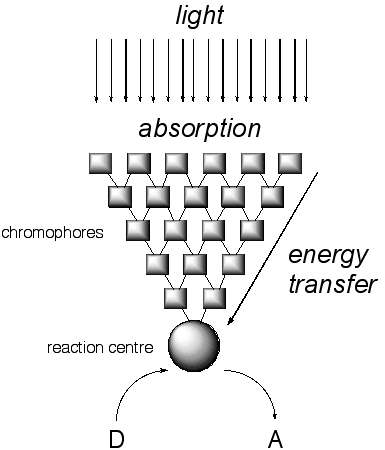
Figure
1 Schematic representation of a light-harvesting complex and reaction
centre
Figure 1 gives an overview of the research we are conducting in artificial
photosynthesis. Energy in the form of light is collected by a series of
chromophores that absorb light of progressively longer wavelength (lower
energy) at each successive level. A large number of chromophores at each
energy level increases the probability of light absorption and with proper
placement of the chromophores, the excitation energy "flows downhill",
where it is collected at a single spot, the reaction centre. Electron
transfer to an electron acceptor (A) creates an initial charge separation.
Subsequent transfer of an electron from an electron donor (D) to the reaction
centre creates the final charge-separated state. The electron and corresponding
"hole" formed by the loss of an electron may then be used for
chemical reactions, be it the production of ATP and O2 in natural
systems, or H2 and O2 in artificial systems. The benefits
of both natural and artificial systems are clear: sunlight is converted
into useful forms of energy. Our approach for the creation of functional
artificial photosynthetic systems is based on coordination and supramolecular
chemistry.
So how do the initial steps
of photosynthesis work in natural systems?
Using photosystem II (PSII) as an example, it is very similar to what
is described above (Fig. 2).1,2
The chromophores are beta-carotenoids (polyalkenes), and chlorophylls
a and b (porphyrinogens). The various chain lengths (carotenoids) and
substituents (chlorophylls) give rise to chromophores that absorb light
of different wavelengths. These chromophores are arranged in such a way
that energy migration to the reaction centre is possible.
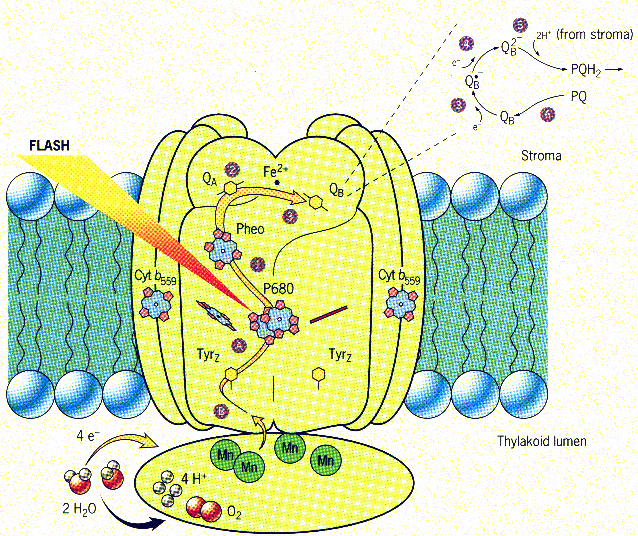
Figure 2 Schematic representation of Photosystem II (PSII)3
The key component of the reaction centre consists of a pair of chlorophylls,
P 680 (Fig. 2, A). Upon excitation of P680, initial electron transfer
(eT) to another chlorophyll molecule, pheophytin, takes place (Fig. 2,
1). Subsequent eT to a quinone acceptor (Qa, Fig. 2, 2) is followed by
another eT to a transient quinone (Qb, Fig. 2; 3), which is reduced (Qb,
Fig. 2; 4 - 6) and subsequently takes this electron out PSII and on to
the production of ATP. On the donor side, a tyrosine residue initiates
et to P680 (Fig. 2, B), which is followed rapidly by eT from a manganese-based
cluster to the oxidized tyrosine residue. The Mn cluster is oxidized four
times in succession before being reduced by H2O and evolving O2.
Thus, energy collected in the LHC is eventually converted to O2
and ATP. This conduit of electron and corresponding "hole" away
from P680 is the key to avoiding charge recombination. So the challenges
are replete; what is the best way to collect light energy, how is it transferred,
what electron acceptors (A) and donors (D) should be used, how is a usable
form of energy finally produced? Although we can only dream of duplicating
the structure that Nature uses in PSII, we can aspire to duplicate its
function.
|


